1. Introduction
Parameters to affect atmospheric corrosion can be div-ided into chemical and physical factors [1-9]. Chemical factors include oxygen, ozone, moisture, sulfur dioxide, salt, dust, acid rain, inclusion on the surface, and other gases. Physical factors are mainly temperature, wind in-tensity, and sunlight. These factors may be changeable with seasons and the natural environment, and these cli-mate changes influence the corrosion behavior of metals and alloys. In general, the environments in which the met-als and alloys are applied can be classified into coastal, industrial, urban, and rural areas [9-13]. However, it should be noted that the above classification is greatly simplified. The applied environment can have a large ef-fect on the lifespan of every metal and alloy, and thus the estimation of lifespan needs to fully understand and take into account the environment [14].
When metallic materials are used or exposed outdoors, degradation can take place by the natural environment, such as sunlight, humidity, rain, dew condensation, and pollutant gases in the air, and thus weather resistance, cor-rosion resistance, and durability are lowered. Therefore, in order to measure the properties in the air, the optimum method is the atmospheric outdoor exposure test [15-18]. The atmospheric outdoor exposure test evaluates the effect of the environmental factors (Cl-, CO, NOx, SOx, O3) in-cluding weather factors (temperature, humidity, quantity of solar radiation, snow, and rain) on the degradation of industrial products (automobile, train, tire, bridge, road facilities, metals, textile, rubber, antenna, cables etc.) that are used or installed at outdoor sites. Because the outdoor exposure test is one of the essential reliability evaluation methods to improve the quality, and estimate the lifespan of new materials or products, it is considered to be very important. Recently, our group reported the atmospheric corrosion of galvanized steels in Korea [19,20]; when the exposure time was increased, the content of Zn from gal-vannealed steel - GA surface decreased while the contents of iron and oxygen tended to increase [19]. With increas-ing exposure times, the galvannealed steel - GA specimen became blackened by the formation of zinc oxide, and red coloration was increased by the formation of red rust. As the exposure time of galvanized steel - GI specimen increased, the surface proceeded to blacken, but no red rust was formed and the color did not change significantly. Regardless of the outdoor exposure area or the specimen, longer exposure times led to lower glossiness, and this be-havior appears to be influenced by the formation of zinc oxide [20]. Even though the research about the atmospheric corrosion of galvanized steels has been continued for a long time, there is little method to predict the lifespan of the steel used in outdoor environments.
Therefore, this work performed the outdoor exposure test of galvanized steel. Two of the exposure sites repre-senting the rural and coastal environments were selected to develop the methodologies to predict the outdoor ex-posure lifespan of galvannealed steel. Two kinds of pre-diction method were induced by the electrochemical and the chemical approaches.
2. Experimental Methods
2.1 Outdoor exposure test
The test material is the galvannealed steel - GA (Zn-Fe). The exposure sites are 2 locations that represent the rural (Andong) and coastal (Busan) environments in Korea. The exposure durations are (1, 6, 12, 24, and 36) months. After the test, the corrosion rate was calculated through weight loss measurement, after the removal of corrosion products or inclusions. Chemical cleaning was performed according to KS D ISO 8407 [22], including 1st step (4 min im-mersion in 70 °C, 10% ammonium chloride solution) and 2nd step (5 min immersion in 25 °C ammonium persul-fate).
2.2 Anodic polarization test
Specimens were cut to a size of 1.5 mm × 1.5 mm and exposed 1 cm2 of area using a flat cell. A polarization test was performed using a potentiostat (DC 105, Gamry Instruments), the reference electrode was a saturated calo-mel electrode (SCE), and the counter electrode was Pt wire. Test solution was simulated acid rain for rural envi-ronment (1.2 mL HNO3 + 1.73 mL H2SO4 in 1 L distilled water and pH 5 was controlled using 10% NaOH) and chloride added simulated acid rain for coastal environment (5% NaCl + 1.2 mL HNO3 + 1.73 mL H2SO4 in 1 L distilled water and pH 5 was controlled using 10% NaOH). Test temperature was room temperature. The test solution was deaerated using N2gas (200 mL/min at 30 min). The scanning rate was 0.33 mV/sec.
2.3 Potentiostatic dissolution test
Specimens were cut to a size of 1.5 mm × 1.5 mm and exposed 1 cm2 of area using a flat cell. The potentio-static dissolution test was performed on +200 mV (SCE), and various diluted acid rains were used. The reference electrode was a saturated calomel electrode (SCE), and the counter electrode was Pt wire.
2.4 Chemical cyclic corrosion test
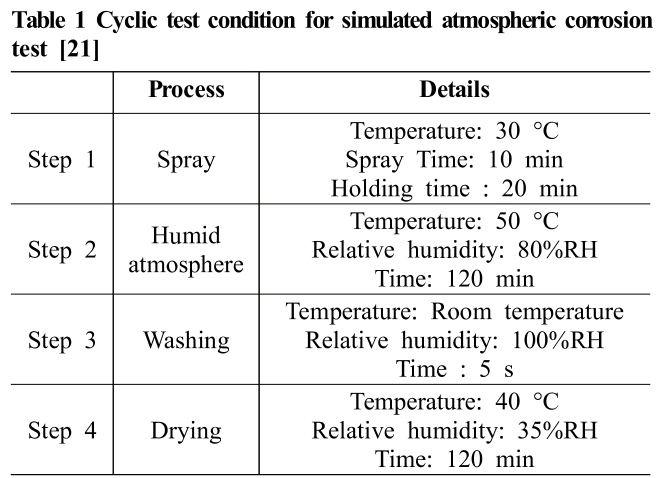
The chemical cyclic corrosion test was performed using a salt sprayer tester and constant temperature/constant hu-midity tester. One cyclic test includes spray, humid atmos-phere, washing, drying process, and the detailed test con-dition is shown in Table 1. Test solution was chloride added simulated acid rain (5% NaCl + 1.2 mL HNO3 + 1.73 mL H2SO4 in 1 L distilled water and pH 5 was con-trolled using 10% NaOH). Dilution ratio was 1:2.
Table 1 Cyclic test condition for simulated atmospheric corrosion test [21]
 2.5 Surface analysis
2.5 Surface analysis
After the exposure test, the corrosion rate was calculated through weight loss measurement, after the removal of corrosion products or inclusions. Chemical cleaning was performed according to KS D ISO 8407 [22], including 1st step (4 min immersion in 70 °C, 10% ammonium chloride solution) and 2nd step (5 min immersion in 25 °C ammonium persulfate). Chemical composition on the surface after chemical cleaning was analyzed using a SEM-EDS (VEGAIILMU, Tescan). After potentiostatic dissolution test and chemical cyclic corrosion test, chem-ical composition on the surface was also analyzed using a SEM-EDS.
3. Results and Discussion
3.1 Electrochemical approach to induce the lifespan prediction of galvannealed steel
In order to induce the lifespan prediction equation, ano-dic polarization and potentiostatic dissolution tests were performed. For test solution, simulated acid rain for rural environment and chloride added simulated acid rain for coastal environment were used since atmospheric corro-sion could be affected by Cl- and SO2/SO42-. These experi-ments aim to find the relationship between outdoor ex-posure test and electrochemical test.
Test material was commercial galvannealed steel – GA. The simulated acid rain for rural area [‘rural’] was (1.2 mL HNO3 + 1.73 mL H2SO4)/1 L, its pH was controlled as 5 using 10% NaOH and the chloride added simulated acid rain [‘coastal’] was (5% NaCl + 1.2 mL HNO3 + 1.73mL H2SO4)/1 L, its pH was controlled as 5 using 10% NaOH.
Fig. 1 shows the anodic polarization curves of galvan-nealed steel in simulated acid rain and chloride added si-mulated acid rain with various dilution ratio. With the ano-dic polarization, anodic current density was increased and the current densities of chloride added simulated acid rain were larger than those of simulated acid rain, regardless of dilution ratios. No solution could be used to induce the prediction equation because of very fast dissolution rate.
Fig. 1 Effect of dilution ratio on the anodic polarization behavior of galvannealed steel - GA in simulated acid rain for rural area and chloride added simulated acid rain for coastal area; (a) no dilution, (b) dilution ratio 1:100, (c) dilution ratio 1:50, (d) dilution ratio 1:25.
Fig. 2 reveals the potentiostatic dissolution test of gal-vannealed steel. Potentiostatic dissolution was performed at +200 mV(SCE) with anodic dissolution time in simu-lated acid rain (Rural and Coastal areas). Fig. 2a is for the dilution ratio 1:50 and Fig. 2b is for the dilution ratio 1:25. With anodic dissolution time, it can be observed that the current density takes step-down after a constant current; In the case of the dilution ratio of 1:50, the step-down was observed after 45,000 seconds for ‘rural’ and was observed after 15,000 seconds for ‘coastal’. In the case of the dilution ratio of 1:25, the step-down was observed after 24,000 seconds for ‘rural’ and was ob-served after 8,000 seconds for ‘coastal’. Surface analysis at each time confirmed that this step-down was induced by the dissolution of iron in the matrix because the Zn-Fe coating was thoroughly dissolved. Among 3 kinds of dilu-tion ratio’s solution, the dilution ratio of 1:25 after lots of preliminary tests was chosen to find the relationship between outdoor exposure test and electrochemical test.
Fig. 2 Corrosion current density of galvannealed steel – GA with potentiostatic dissolution time at +200 mV(SCE) in simulated acid rain for rural area and chloride added simulated acid rain for coastal area; (a) dilution ratio 1:50, (b) dilution ratio 1:25.
Fig. 3 shows the effect of test time on the Zn content of the surface of galvannealed steel by the outdoor ex-posure test and the anodic dissolution test in simulated acid rain with the dilution ratio of 1:25. Fig. 3a is for the rural area – Andong and Fig. 3b is for the coastal area –Busan. In the figures, trend line and trend equation were shown. The surface analysis and surface appearance after the outdoor exposure test were already reported in other paper [19,20] and we plotted Zn contents with differ-ent style in Fig. 3. It is interesting that Zn contents with potentiostatic dissolution time were similar to those of out-door exposure test with exposure time.
Fig. 3 Effect of test time on the Zn content of the surface of galvannealed steel - GA by the outdoor exposure test and the anodic dissolution test in simulated acid rains with dilution ratio of 1:25; (a) rural area, (b) coastal area.
From two trend equations, we can obtain the time to show the same Zn content in the outdoor exposure test and potentiostatic dissolution test. Fig. 4 was plotted from this procedure. Fig. 4 shows the relationship between ano-dic dissolution time and outdoor exposure time to reveal the same Zn content on the surface of galvannealed steel. Fig. 4a is for the rural area and Fig. 4b is for the coastal area. As shown in figures, two lifespan prediction equa-tions were derived.
Fig. 4 Relationship between anodic dissolution time and outdoor exposure time to reveal the same Zn content on the surface of galvannealed steel - GA; (a) Rural area, (b) Coastal area.
For rural area,
Outdoor exposure time (months) = 0.10 × potentiostatic dissolution time (minutes) – 1.04.
For coastal area,
Outdoor exposure time (months) = 0.16 × potentiostatic dissolution time (minutes) – 0.70.
3.2 Chemical approach to induce the lifespan prediction of galvannealed steel
Another approach for deriving the lifespan prediction equation was the chemical cyclic corrosion test. Fig. 5 shows the effect of test time on the Zn content of the surface of galvannealed steel - GA by the outdoor ex-posure test and the cyclic corrosion test for the coastal area. The outdoor exposure durations were (1, 6, 12, 24, and 36) months and after each exposure duration, chemical composition was analyzed on the surface. The cyclic cor-rosion test solution was chloride added simulated acid rain with the dilution ratio of 1:2. After 1, 3, 6, 9, 11 cyclic tests, chemical composition was analyzed on the surface. As shown inFig. 5, a similar trend was observed in the outdoor exposure test and cyclic corrosion test, and two trend equations were also shown in the figure.
Fig. 5 Effect of test time on the Zn content of the surface of galvannealed steel - GA by the outdoor exposure test and the cyclic corrosion test for coastal area.
From two trend equations, we can obtain the time to show the same Zn content in the outdoor exposure test and a cyclic corrosion test. Plotting was done with this pro-cedure in Fig. 6. Fig. 6 shows the relationship between cy-clic corrosion test cycle and outdoor exposure time to reveal the same Zn content on the surface of galvannealed steel for the coastal area. As shown in figures, a lifespan pre-diction equation for the coastal environment was derived.
Outdoor exposure time (months) = 1.94 × cyclic corro-sion test (cycles) – 0.65.
Fig. 6 Relationship between corrosion test cycles and outdoor exposure time to reveal the same Zn content on the surface of galvannealed steel – GA for coastal area.
4. Conclusions
This work performed the outdoor exposure test of gal-vannealed steel - GA. Two of exposure sites representing the rural (Andong) and coastal (Busan) environments in Korea were selected to develop the methodologies to pre-dict the outdoor exposure lifespan of galvannealed steel. Two kinds of prediction method were induced by the elec-trochemical and chemical approaches;
(1) In order to find out the atmospheric corrosion life-span by outdoor exposure test, the very long test time is needed. However, accelerated electro-chemical and chemical methods can be induced by the selection of electrochemical condition or chem-ical cyclic test condition with the combination of the appropriate test solution.
(2) Using a potentiostatic dissolution test in simulated acid rain for rural or coastal environments, two life-span prediction equation were derived; For rural area, “Outdoor exposure time (months) = 0.10 × potentiostatic dissolution time (minutes) – 1.04” and for coastal area, “Outdoor exposure time (months) = 0.16 × potentiostatic dissolution time (minutes) – 0.70”
(3) Using a chemical cyclic corrosion test in simulated acid rain for the coastal environment, a lifespan pre-diction equation for coastal environment was de-rived; “Outdoor exposure time (months) = 1.94 × cyclic corrosion test (cycles) – 0.65”
Acknowledgement
This work was supported by a grant from the 2019–20 Research funds of Andong National University.
References
- W. H. Vernon, T. Faraday Soc., 23, 113 (1927). https://doi.org/10.1039/tf9272300113
- W. H. Vernon, T. Faraday Soc., 27, 255 (1931). https://doi.org/10.1039/TF9312700255
- W. H. Vernon, T. of Faraday Soc., 31, 1668 (1935). https://doi.org/10.1039/TF9353101668
- N. A. Lange, Handbook of Chemistry, 10th ed., McGrawill, New York (1961).
- I. Suzuki, Corros. Eng., 30, 639 (1981). https://doi.org/10.3323/jcorr1974.30.11_639
- T. Murata, Corros. Eng., 33, 598 (1984).
- B. N. Popov, Corrosion Engineering : Principles and Solved Problems, p. 452, Elsevier, New York, (2015).
- M. G. Fontana, Corrosion Engineering, 3rd, p. 372, McGraw-hill, New York (1986).
- Z. Ahmad, Principles of Corrosion Engineering and Corrosion Control, p. 550, Elsevier, New York (2006).
- P. R. Roberge, Corrosion Engineering: Principles and Practice, p. 330, McGraw-hill, New York (2008).
- S. Syed, Emirates Journal for Engineering Research, 11, 1, (2006).
- J. Alcantara, B. Chico, I. Diaz, D. de la Fuente, and M. Morcillo, Corros. Sci., 97, 74 (2015). https://doi.org/10.1016/j.corsci.2015.04.015
- M. Natesan, G. Venkatachari, and N. Palaniswamy, Corros. Sci., 48, 3584 (2006). https://doi.org/10.1016/j.corsci.2006.02.006
- S. K. Chang, J. Kor. Inst. Surf. Eng., 30, 69 (1997).
- W. Han, C. Pan, Z. Wang, and G. Yu, Corros. Sci., 88, 89 (2014). https://doi.org/10.1016/j.corsci.2014.07.031
- E. A. Alvarenga and V. F. C. Lins, Surf. Coat. Technol., 306, 428 (2016). https://doi.org/10.1016/j.surfcoat.2016.04.021
- D. Perssona, D. Thierryb, and O. Karlssona, Corros. Sci., 126, 152 (2017). https://doi.org/10.1016/j.corsci.2017.06.025
- D. To, O. Umezawa, and T. Shinohara, Mater. Trans., 59, 1239 (2018). https://doi.org/10.2320/matertrans.MF201702
- K. T. Kim and Y. S. Kim, Corros. Sci. Tech., 17, 301 (2018).
- K. T. Kim and Y. S. Kim, Corros. Sci. Tech., 17, 231 (2018).
- ISO 14993, Accelerated testing involving cyclic exposure to salt mist, dry and wet conditions (2015).
- KS D ISO 8407, Corrosion of metals and alloys - Removal of corrosion products from corrosion test specimens (2014).