1. Introduction
In the automotive fuel tank market, plastic materials are dominant, but steel materials are slightly recovering new competitiveness due to the emergence of hybrid ve-hicles and the enactment of global environmental regu-lations [1]. Fuel tank materials are undergoing a major change from four perspectives. The enactment of the Euro-VI environmental regulations on the basis of zero emissions, the increase in biofuel consumption, the light weight for improving fuel efficiency of automobiles, and the cost reduction strategy of automobile companies, are the driving force of change. From this point of view, steel materials are advantageous in terms of environmental friendliness and price, however plastic materials have ad-vantages in terms of corrosion resistance and light weight as well as plastic molding [2].
Since the fuel tank steels is related to the safety of the human body, it shall have the following characteristics for long-term use of the vehicle. First, it is corrosion re-sistance to fuel. A warranty of more than 10 years is usu-ally required, as the purchase of a car is subject to semi-permanent use. Recently, the importance of corro-sion of materials has been emphasized as the use of bio-fuels has increased to reduce the use of fossil fuels. Second, various welding characteristics, such as seam, spot, arc, and projection, shall be excellent for the manu-facture of the fuel tank. Generally, galvanized steels are coated with organic-inorganic layer on the surface to en-hance corrosion resistance, resulting in increased electrical insulation and poor weldability. The third is press-forming ability. Deep drawing property of steels are required to suit the complex structure of a vehicle and to maximize the fuel capacity. Although mild steel with excellent draw-ing property is being used for this purpose, but the applica-tion of high-strength steel to make the weight reduction of car body has become more important. Fourth, it is cor-rosion resistance on the exterior of fuel tank. The car is operated in various environments, so the exterior of fuel tank is painted. It shall have characteristics that comply with customer's painting conditions such as electro-deposition, foam or powder painting.
The plating steels for fuel tank in POSCO was first developed and produced in 1990. Terne (Pb-8%Sn plating) steels, which was the first generation product, was pro-duced until 1998, but was replaced by Zn-Ni plating steels, which was named Pb-free under heavy metal regulations. The Zn-Ni plating steels, made of both Cr(VI) primer and conductive top layer to enhance corrosion resistance, were produced by 2007. Then by the enactment of the environ-mental control act on Cr(VI) component, the organic-in-organic thin layer coated Zn-Ni plating steels were devel-oped in 2007 and has been produced so far [3]. Recently, we developed a new functional composite coated product in accordance with the needs of our customers to improve productivity at welding line.
2. Experimental
2.1. Nano-composite Coating Solution
Nano composite coating solution was prepared by pre-cisely dispersing nano-material into polymeric composite composition in aqueous solution. The polymeric compo-site composition was prepared by adding anti-corrosion and fluidity enhancers to the acryl modified polyurethane in aqueous solution. And nano-material was produced by dispersive surface modification of nano-sized metal pow-der (average diameter, 70 nm) with acrylate oligomer at 3000 rpm stirring for 24 hours by using ultrafast ball-mill diffuser (VMX Dispermat). The nano-composite solution was precisely prepared and adjusted the solid content to 14 ± 1 % and the viscosity of the Brookfield to 8.0 ± 0.1 cps by adding water, and then stirred for 24 hours.
2.2. Nano-composite Coated Steel Sheet
The nano-composite coated steels were manufactured as following. Firstly Zn-Ni plating (Ni 11 ± 1 %, plating weight 30 ± 2 g/m2) was produced through horizontal elec-troplating cell after cleaning the cold rolled steels with alkali degreasing, acid cleaning, and water cleaning proc-ess on the electrogalvanized line. Nano-composite coated steels are manufactured so that the coating weight is a range of 0.7 to 1.1 g/m2 on the surface of the plating steels by use of roll coater and then hardening it in the induction furnace for 20 seconds at a temperature of 180 ± 5 °C. The coating amounts were measured by a portable Near-IR meter or wet method using a pre-written calibration.
2.3. Evaluation Methods and Instrumentation
Evaluation of surface properties of nano-composited steel sheets were performed using the following methods and instruments. As comparative purposes, both Zn-Ni plating steels coated with conventional composite coating (plating weight, 30 ± 2 g/m2, 1.2 ± 0.1 g/m2) and Sn-Zn plating steels (plating weight, 40 ± 2 g/m2) were used. Corrosion resistance was evaluated for flat- and X-cut panels up to the time of white and red rust appearance in the salt spray test (SST), after cutting the specimen to a size of 70 × 140 mm [4]. The durability for the fuel is evaluated in the following ways: The cup drawn speci-men for simulating the shape of the fuel tank was manu-factured by cutting the steelsto 110mm∮ in size and processing it with a punch diameter of 50 mm, drawing height of 30 mm and a radius of curvature (punch R = die R = 6R) using a universal test machine. The fuel dura-bility was evaluated byputting fuel of 30 mL into the drawn cup and then analyzing the corrosion appearance inside the cup and the amount of metal dissolved in the residual fuel after shaking it at 60 rpm in given conditions. (conditions; 1000 hours at 50 °C temperature for gasoline and 8 weeks at 80 °C temperature for diesel) The variety of fuel compositions for test were prepared by using gaso-line and diesel fuel, biofuels (bioethanol, biodiesel), corro-sive acid compounds and salts, and evaluated to accelerate corrosion in fuel tanks due to aging.
The seam and spot weldability evaluations of nano- composite coated steels were performed as follows. High speed seam welder used in inverter DC welder (Hyosung, Ltd.) and evaluation conditions were 8 mm in electrode diameter, 6 kN in loading pressure, 6 mpm in weld speed, 33 ms in electricity generation time, and 10 ms in resting time. Spot weldability was achieved using the above weld-er under conditions of 6 mm in electrode diameter, 2.3 kN in loading force, 200 ms in electricity generation time and 3.58 mm in reference button diameter. Friction co-efficients and limit drawing ratios were evaluated to assess the press-formability of nano-composite coated steels. The friction coefficient is assessed by sliding 100 mm at the speed of 1000 mm/min with a loading force of 600 kgf (5 MPa) without treatment of press oil [5]. After making a circular blanks of 95, 100, 105, 110, 115, and 120 mm, cup was applied a diameter of 50 mm (shoulder radius = 8 mm), a diameter of 52.6 mm (shoulder radius = 8 mm), a speed of 1.6 mm/s, and a blank holder force (BHF) under 20 kN conditions, respectively. When the drawing ratio (blank diameter/punch diameter) increases and the maximum molding load is reached when fracture occurs, the drawing ratio is determined as the limit drawing ratio.
3. Results and Discussions
Generally the materials for fuel tank are installed in the human-driven vehicles, so durability of the materials are of the utmost importantance. In particular, galvanized steels undergo additional surface treatment on the surface of plating steels to enhance corrosion resistance, as corro-sion should not occur for more than 10 years. Surface treatment to prevent corrosion causes poor welding char-acteristics in the manufacture of the fuel tank by coating organic-inorganic composite resin, mainly composed of non-conductive materials. Therefore, coating technology with possible thin layer coating is being developed to en-hance the corrosion resistance of the galvanized steels, but it is judged that it is not satisfactory.
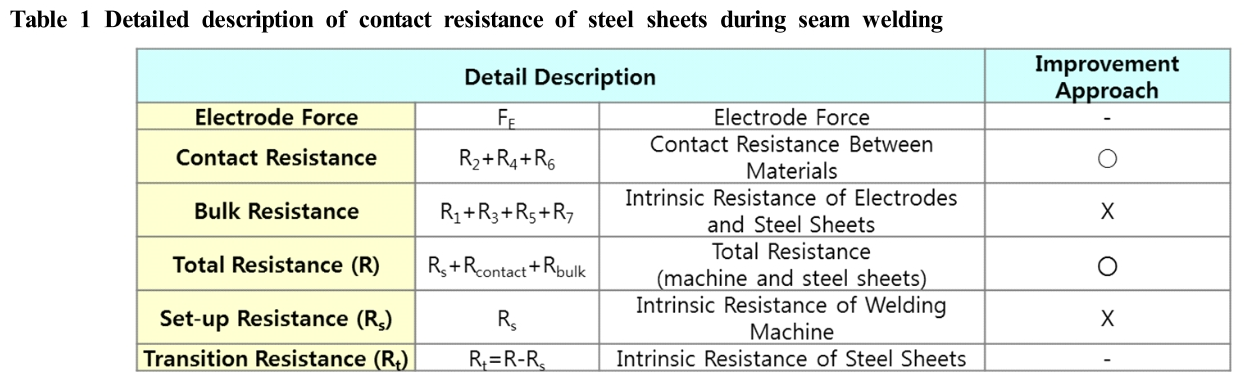
Manufacture of the fuel tank is completed by press- forming and then joining of the top and bottom sheets together by seam welding, together with a spot welding between the lower sheet and baffle to prevent the fuel from fluctuation. When the joint is incomplete during seam welding, it not only creates a problem of water leak-ing during the hydraulic test, but also affects productivity. Therefore, methods are required to improve the electrical conductivity in a composite coating solution. In particular, the contact resistance during seam welding can be ex-pressed as R2+R4+R6, a resistance that forms the contact surface of the steels, as shown in Fig. 1 and Table1. The conventional composite coating layer consists of non-conductive resin with electrical insulation, which is believed to increase the contact resistance (R4), resulting in poor weldability [6]. Therefore, the coating layer (R4) which corresponds to the inside of the fuel tank needs to be improved, thereby improving the melting and joining of the steel material. Meanwhile, the contact surface (R2, R6) corresponding to the outside of the fuel tank is consid-ered sufficiently conductive by the pressurized force (6 kN) of the welding rod, and thus the conventional compo-site coating was applied.
Fig. 1 Schematic diagram of contact resistance in the interfacial between steel sheets during seam welding.
Table 1 Detailed description of contact resistance of steel sheets during seam welding
 3.1. Nano-composite Coated Steel Sheet
3.1. Nano-composite Coated Steel Sheet
The composite coating for the fuel tank is not only ex-cellent corrosion resistance even though thin film coating, but also chemical resistance to the fuel. Conventional composite coating solutions include corrosion-resistant ad-ditives such as silica in polymeric urethane resin solution. In this study, nano-composite coating solutions were pre-pared by mixing composite resin solution and nano-size conductive materials to improve electrical conductivity by use high speed bead-mill dispersion technology. Although conductive materials are generally higher gravity than non-metallic polymers, their surface tension between the particles is significantly reduced when the 70 nm size of nanomaterial is distributed to organic surfactant by surface modification, thus improving the dispersion stabilization within the solution. The development solution is an organ-ic-inorganic composite coating solution containing nano-material, and is designed to have a 4 : 6 ratio of organic to inorganic compounds for excellent corrosion, chemical resistance, and heat resistance. The above nanomaterial dispersed solution was mixed with a composite coating solution to prepare onecomponent nano-composite coat-ing solution. The manufactured solution is coated by roll coater and the coating is treated as 0.7 to 1.1 g/m2 on the surface of the plated Zn-Ni steel plate, and is dried at the PMT = 180 °C and then nano-composite coated steel sheet, as shown in Fig. 2 is manufactured.
Fig. 2 Conceptual depiction for the coating layer structures of new nano-composite coated steels and conventional steels.
3.2. Quality Characteristics
As mentioned earlier, the coating weight of composite coating layer coated on the surface of galvanized steels significantly affects corrosion, weldability and press- formability. The developed nano-composite coating was evaluated for the coated steel sheets of a range of coating weights with excellent corrosion resistance to fuel. In oth-er words, it was judged that treating the coating at 0.7 to 1.1 g/m2satisfies the customer's required quality in terms of corrosion and fuel durability. All of the following quality evaluations are a result of steel sheets having a coating weight of 0.9 ± 0.1 g/m2.
3.2.1. Corrosion Resistance
The evaluation of the corrosion resistance of salt spray for nano-composite coated steels were compared to the conventional composite coated steels and Sn-Zn plating steels used for fuel tank use. In Fig. 3, in the evaluation of the flat- and X-cut panels, nano-composite steels showed an improvement in corrosion resistance when comparing the time of white rust generation with the con-ventional composite coating. Meanwhile, the salt spray test on the X-cut area of Sn-Zn plating steels showed red rust on the surface after 480 hours or more. These results are judged to be due to the inferior sacrificial property of the Sn-Zn plating steels compared to the Zn-Ni plating steels, which is highly likely to cause severe corrosion in the spot or seam weld region after manufacture of the fuel tank. Similarly, in Fig.4, the evaluation of the cup drawn unit showed similar results. Nano-composite coated steels showed similar corrosion patterns in the range of 0.7 to 1.1 g/m2coating weight and showed a significant improvement in their sacrifice to the cup drawn parts com-pared to conventional composite coating. These results are judged to have improved self-healing properties of nano-material and inorganic compounds contained in com-posite coating layer.
Fig. 3 Evaluation on the corrosion resistance in flat- and X-cut panels of new nano-composite coated steels.
Fig. 4 Evaluation on the corrosion resistance of cup drawn specimen according to the coating weights of new nano-composite coated steels.
3.2.2. Fuel Durability
Fuels for motors, especially in European and North American countries, have recently increased the use of biofuels to prepare for the depletion of fossil fuels. Gasoline uses mainly a mixture of corn based bioethanol (E10 to E30), but diesel fuels tend to use a mix of bio-diesel (BD5 to BD20) such as soybean oil and palm oil. The corrosion resistance in fuel was conducted by simulat-ing the fuel tank, forming a steels in a cup shape, adding fuel and corrosion acceleration additives, and shaking the cup at 60 rpm in the shaking chamber during 1,000 hours for gasoline and 8 weeks (1344 hours) for diesel. In the assessment of gasoline as shown in Fig. 5, the develop-ment nano-composite coated Zn-Ni steels did not differ significantly from the comparison materials at the rate of corrosion at which the addition of bioethanol was changed, but with the addition of formic acid as a corrosion en-hancer, they were less corrosive than with the conven-tional steels. Especially when the condensed water in the fuel tank was not added, corrosion was much more severe than when the water was added. If formic acid is mixed in water, the free acid is diluted and the concentration is relatively low, thus slowing the rate of corrosion. In particular, the severe corrosion appearance in the composi-tion of E0 without adding bioethanol is believed to be related to the concentration of free acid. This is because as the addition of bioethanol increases, the concentration of the free acid decreases, slowing the reactivity of the steel surface. On the other hand, it is believed that the red rust appearance for the Sn-Zn plating steels, which was used as a comparative material, was promoted by lo-cal corrosion due to the inferior sacrificial properties. On the other hand, those steels in Fig. 6 show generally good resultsfor all of fuel compositions of diesel fuel and bio-diesel fuel and corrosive additives. The result is that gaso-line fuel (C5 to C7, carbon number in unit molecule) has a smaller molecular weight than diesel (C10 to C18), which is considered to be a result of active molecular movement along with free acid. Analysis of Zn and Fe metals from solution after evaluation in all fuel composi-tions showed that nano-composite coated steel sheet per-formed better than conventional composite coating.
Fig. 5 Evaluation of the fuel durability in the composition of gasoline and bioethanol for the new nano-composite coated steels.
Fig. 6 Evaluation of the fuel durability in the composition of diesel and biodiesel for the new nano-composite coated steels.
3.2.3. Weldability
The weldability of the fuel tank steel plate is closely related to the electrical conductivity of the surface. Organic-inorganic coatings treated to enhance corrosion resistance on the surface of Zn-Ni plated steels shall have good fuel durability on the interior and good paint adhe-sion on the exterior. Thus, the developed nano-composite coated steels were applied to reduce the interfacial contact resistance of the steels, as shown in Fig. 3. As shown in Fig. 7, the result of measuring the resistance of welding was significantly improved to 0.13 mΩ compared to 62 mΩ of the conventional composite coating. This resulted in a similar result to 0.2 mΩ of Sn-Zn plating steels, which are considered to have excellent electrical conductivity on the surface. In addition to improving the seam welding speed required by the customer from 3 mpm to 6 mpm, the optimum weld current range has been significantly improved from 0.7 kA to 1.8 kA, which is well compared with Sn-Zn plating steels. In addition, the spot weldability evaluation showed excellent results with a weldable cur-rent range of 2.0 kA and a continuous welds of 2000. These results are judged to be due to an improvement in electrical conductivity as the resistance of the contact surfaces of the coating layer containing nanomaterial is reduced.
Fig. 7 Comparisons of contact resistance and weldable current range in seam weld for new nano-composite coated steels and conventional steels.
3.2.4. Press Formability
The fuel tank must be constructed to accommodate the complex structure of the car, so that the shape is complex and made from deep drawing processing, which requires good press-forming. For deep drawing forming, it is known that the material's plastic anisotropic coefficient (r value) and the friction characteristics between the mate-rial and the mold are important factors [7]. However, the importance of friction characteristics is increasing as the high strength steel is recently being applied to fuel tanks to reduce the body weight, and the material formability represented by the plastic anisotropic coefficient is being reduced. The plasticity was evaluated by measuring both the friction coefficient and the limit drawing ratio (LDR). The friction coefficient for the front and the back of the development steels were 0.17 ±0.01 for without and 0.125 ±0.005 for with treatment of the rust-proof oil, which was better than 0.25 ±0.01 and 0.155 ±0.005 for the conventional steels, respectively. Also, as shown in Fig. 8, the LDR for nano-composite coated steel sheet was 2.36 under test conditions, which was very good for draw-ing compared to the conventional steels 2.28. A significant improvement in the limit drawing ratio is considered to be due to the improvement of the friction characteristics of the development steels, especially due to the enhance-ment of the lubricants of the coating layer containing nano-size materials.
Fig. 8 Comparison of LDR values for new nano-composite coated steels and conventional steels.
4. Conclusion
The automotive fuel tank is mainly manufactured by seam welding of steel sheets consisting of upper and lower panels. Zn-Ni plating steels with new functional nano- composite coating were developed to enhance the pro-ductivity of seam welding in customers. Nano-composite coated steels are manufactured by roll coating on the sur-face of plating steels with a high temperature hardening of a nano-composite coating solution manufactured by precisely dispersing polymer resin solution and surface modified nano-material.
Developed nano-composite coated steels are not only better than conventional composite steels in their corro-sion resistance and fuel resistance evaluation for a range of gasoline and diesel fuels, but also significantly reduce the contact resistance of the surface during seam welding, improving the weldable current range at high speeds. In addition, the limit drawing ratio value was significantly improved in the formability evaluation. These results are considered to be a result of improved surface electrical conductivity by nano-material contained in the nano-com-posite coating layer and improved lubrication of the coat-ing layer by nano-sized particles. The developed nano- composite coating steels are expected to be applied to au-tomotive fuel tank material, which will greatly contribute to improving productivity of customers.
References
- Evaluation of the Corrosion Durability of Steel Systems for Automotive Fuel Tanks, SASFT Report 2002-2004.
- P. Mould, R. Sheffield, and B. Wilkinson, Durability Performance of Fuel-Tank Steels in Bio-Diesel Fuels, SASFT Reprot (2010).
- Y. S. Jin, Proc. 9th Int. Conf. on Zinc and Zinc Alloy Coated Steel Sheet (GALVATECH 2013), pp. 25 - 33, Metallurgical Industry Press, Beijing, China (2013).
- JIS Z 2371, Methods of Salt Spray Testing, Japanese Standards Association (2000).
- ASTM G-115-98, Standard Guide for Measuring and Reporting Friction Coefficients (2004).
- ISO 18594, Resistance Spot, Projection- and Seam-welding-Method for Determining the Transition Resistance on Aluminum and Steel Material (2007).
- W. F. Hosford and R. M. Caddell, Metal Forming Mechanics and Metallurgy, 3rd ed., pp. 220 - 228, Cambridge University Press, New York (2007).